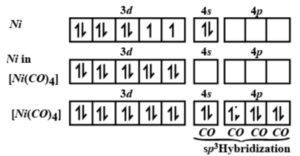
Among [Ni(CO)4], [Ni(CN)4]^2- , [NiCl4]^2- species, the hybridisation states at the Ni atom are, respectively - Sarthaks eConnect | Largest Online Education Community
![The hybridization of ${\\left[ {Nic{l_4}} \\right]^{2 - }}$ and ${\\left[ { Ni{{\\left( {CN} \\right)}_4}} \\right]^{2 - }}$ considering hybridization of the metal ion are respectively.(A) $s{p^3},ds{p^2}$(B) $ds{p^2},s{p^3}$(C) Both $s{p^3}$(D) Both ... The hybridization of ${\\left[ {Nic{l_4}} \\right]^{2 - }}$ and ${\\left[ { Ni{{\\left( {CN} \\right)}_4}} \\right]^{2 - }}$ considering hybridization of the metal ion are respectively.(A) $s{p^3},ds{p^2}$(B) $ds{p^2},s{p^3}$(C) Both $s{p^3}$(D) Both ...](https://www.vedantu.com/question-sets/292d2484-f6d0-482e-82eb-991506f4d8166691884101172465972.png)
The hybridization of ${\\left[ {Nic{l_4}} \\right]^{2 - }}$ and ${\\left[ { Ni{{\\left( {CN} \\right)}_4}} \\right]^{2 - }}$ considering hybridization of the metal ion are respectively.(A) $s{p^3},ds{p^2}$(B) $ds{p^2},s{p^3}$(C) Both $s{p^3}$(D) Both ...
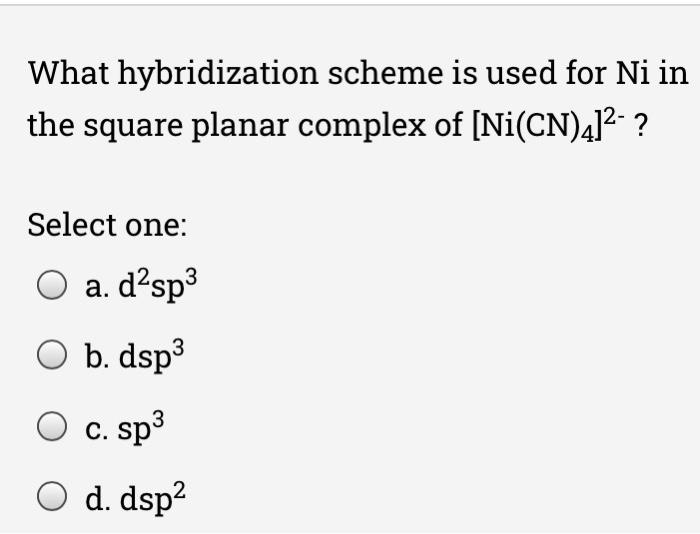
How to find the hybridizationof[Ni(CN)4]2 n ntand the d orbital used in it. What is the hybridization of NO2?
![Write the Hybridization Type and Magnetic Behaviour of the Complex [Ni(Cn)4]2−. (Atomic Number of Ni = 28) - Chemistry | Shaalaa.com Write the Hybridization Type and Magnetic Behaviour of the Complex [Ni(Cn)4]2−. (Atomic Number of Ni = 28) - Chemistry | Shaalaa.com](https://www.shaalaa.com/images/_4:837e04eba2fc4496b5ad1a30a0ede108.png)
Write the Hybridization Type and Magnetic Behaviour of the Complex [Ni(Cn)4]2−. (Atomic Number of Ni = 28) - Chemistry | Shaalaa.com
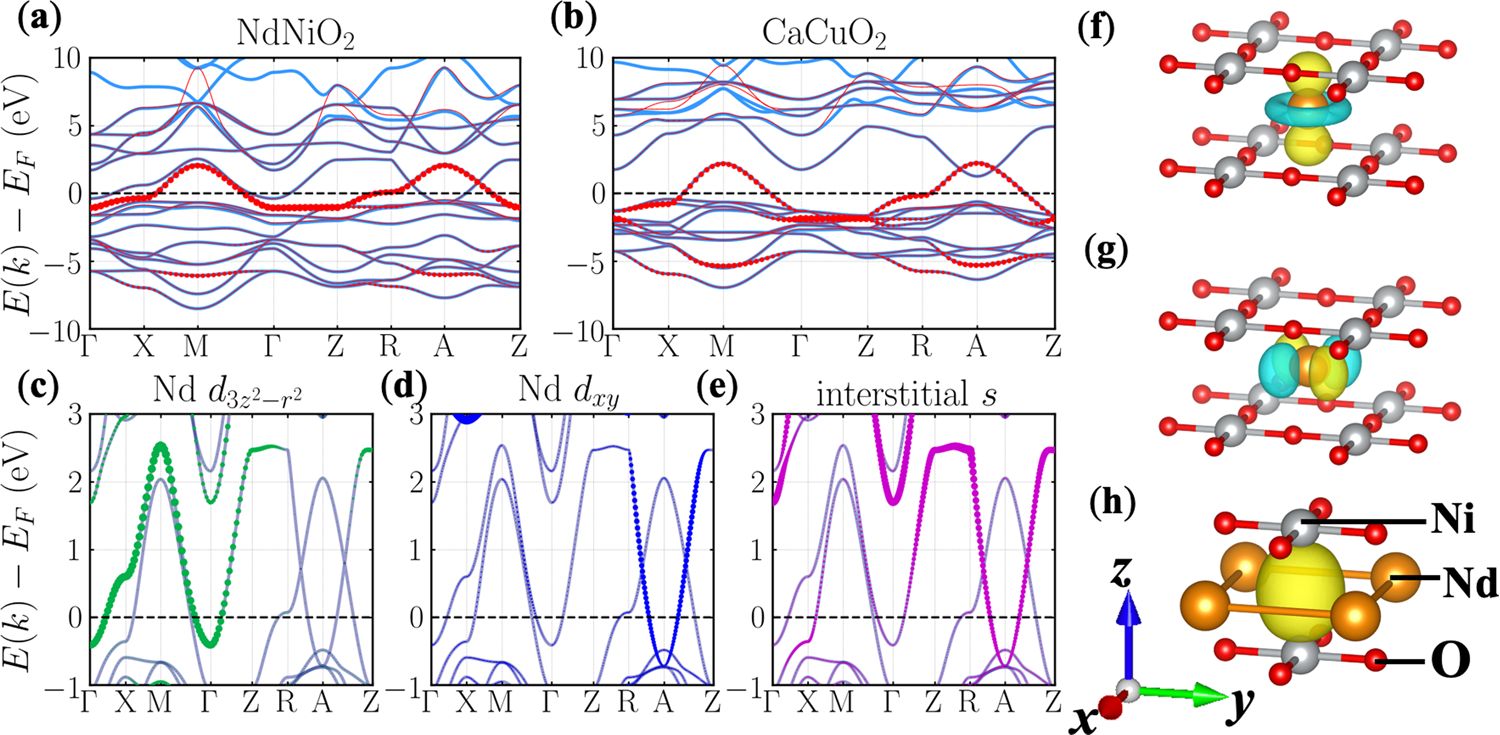
A substantial hybridization between correlated Ni-d orbital and itinerant electrons in infinite-layer nickelates | Communications Physics

Orbital polarization and pd hybridization Ni L3-edge X-ray absorption... | Download Scientific Diagram
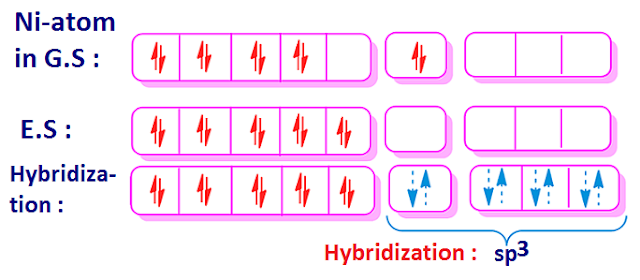
Why is Ni (CO) 4 tetrahedral and diamagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium
![Deduce the structures of [NiCl4]2– and [Ni(CN)4]2– considering the hybridization of the metal ion. Calculate the magnetic moment (spin only) of the species. - Zigya Deduce the structures of [NiCl4]2– and [Ni(CN)4]2– considering the hybridization of the metal ion. Calculate the magnetic moment (spin only) of the species. - Zigya](https://www.zigya.com/application/uploads/images/chen12070397_571494e890adc.png)
![Ni(NH3)6]+3 hybridisation structure - YouTube Ni(NH3)6]+3 hybridisation structure - YouTube](https://i.ytimg.com/vi/iZYGIu-3Fls/maxresdefault.jpg)
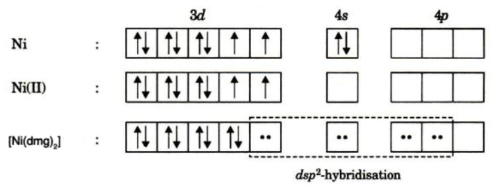
![Ni(CN)4]2-Hybridisation , Geometry and Magnetic nature -coordination compounds - YouTube Ni(CN)4]2-Hybridisation , Geometry and Magnetic nature -coordination compounds - YouTube](https://i.ytimg.com/vi/5S_my6-2Vkc/maxresdefault.jpg)
![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-3.png)

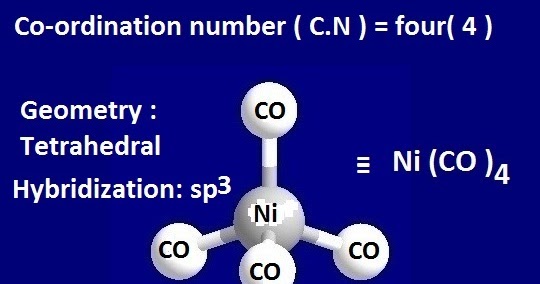
![Among [Ni(CO)4], [Ni(CN)4]^(2-), [NiCl4]^(2-) species, the hybridizati Among [Ni(CO)4], [Ni(CN)4]^(2-), [NiCl4]^(2-) species, the hybridizati](https://d10lpgp6xz60nq.cloudfront.net/physics_images/A2Z_CHM_XII_C09_E01_327_S01.png)
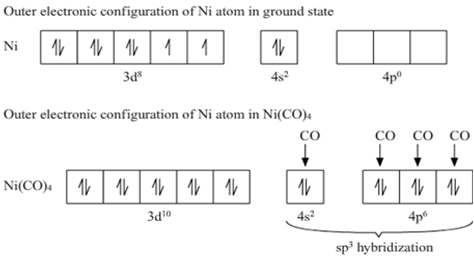
![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-1.png)
![What is the hybridization for [NiCN4]2 ? What is the hybridization for [NiCN4]2 ?](https://byjus-answer-creation.s3.amazonaws.com/uploads/2.14.jpg_img_upload_solution_2022-05-30%2005:07:29.453226.png)
![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-2.png)
![Solved] Hybridisation of [NiCl4]2- is ______. Solved] Hybridisation of [NiCl4]2- is ______.](https://storage.googleapis.com/tb-img/production/21/03/F1_Puja%20J_Anil_02.03.21_D9.png)