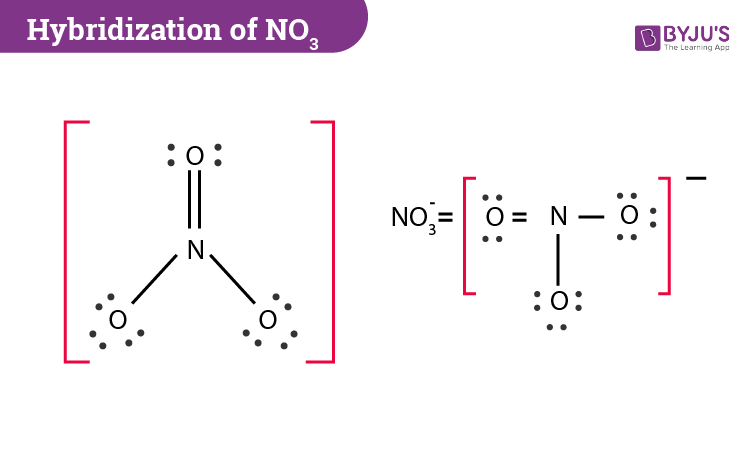
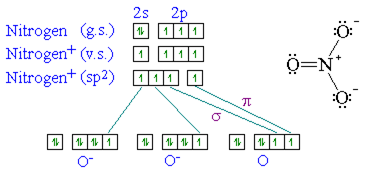
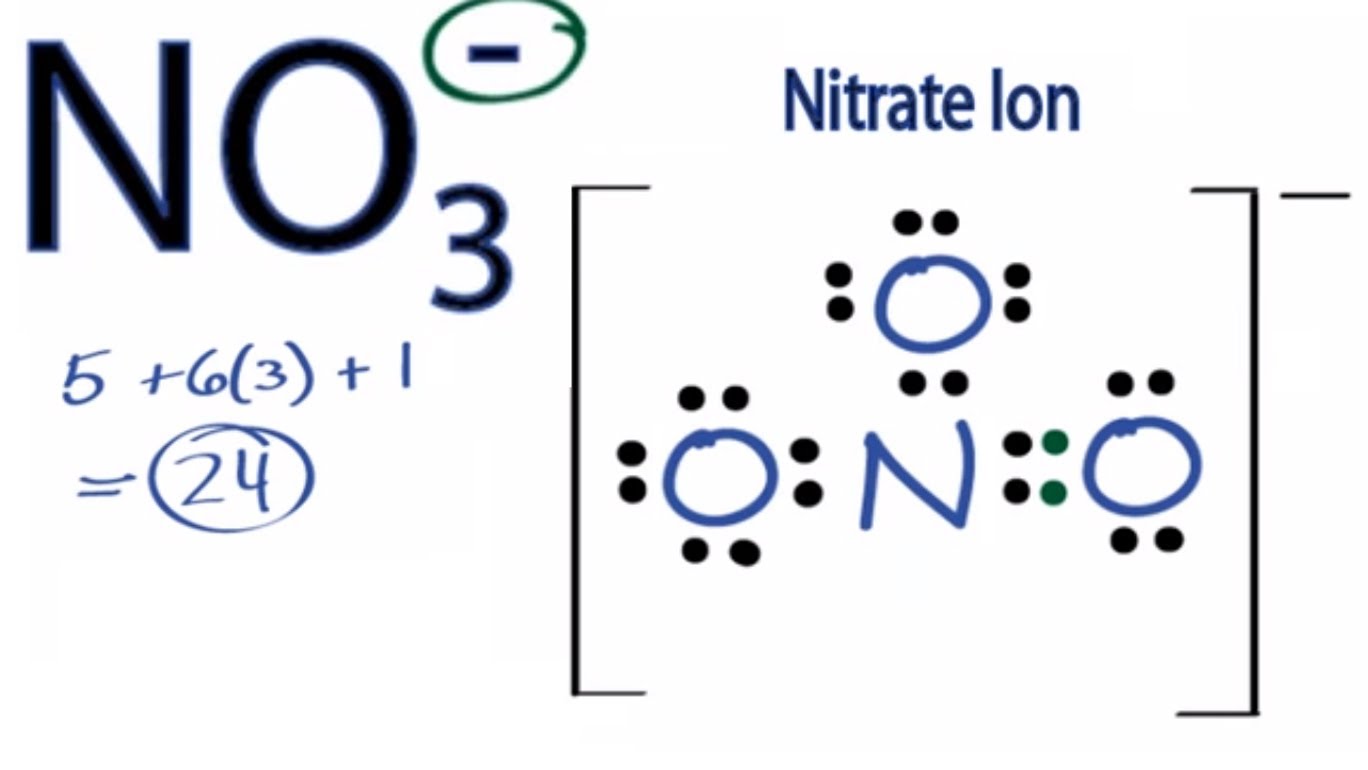
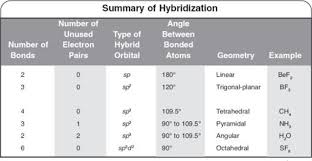
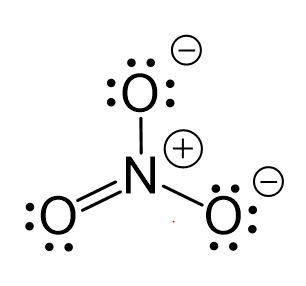
The hybridization of atomic orbital of nitrogen in NO2+, NO3- and NH4+ are:a)sp, sp2, sp3b)sp2, sp3, spc)sp2, sp, sp3d)sp, sp3, sp2Correct answer is option 'A'. Can you explain this answer? - EduRev

Some of the properties of the two species, $NO_{3}^{-}$ and ${{H}_{3}}{{O}^{+}}$ described below. Which one of them is correct?(a) dissimilar in hybridization for the central atom with different structures(b) isostructural with same
The hybridization of orbitals of N atom in NO3^- NO2^+ and NH4^+ are respectively - Sarthaks eConnect | Largest Online Education Community

inorganic chemistry - Hybridization of orbitals and forming of bonds in the nitrogen dioxide molecule - Chemistry Stack Exchange
The hybridisation of orbitals of N atom in NO3^–, NO2^+ and NH4^+ are respectively - Sarthaks eConnect | Largest Online Education Community

The hybridizations of atomic orbitals of nitrogen in NO^+_2, {NO}^-_3 and NH^+_4 are respectively:sp^{2},,sp and , sp^3sp, sp^{2} and , sp^3sp, sp^{3} and , sp^2sp^{2}, sp^3 and sp ,